Understanding the Fractional Distillation Process

To better understand how the fractionation process is accomplished, let Tower Packing Manufacturer show you how it's done in the kitchen:
• You can start by pouring tap water into a pot. Place a glass bowl on top of the water, making sure it floats.
• Turn on the stove burner and place the pot on top of it, bringing it to a boil.
• Continually make sure the glass bowl is floating. If it starts to sink, turn down the heat.
• As the water begins to boil, turn the cover of the pot upside down and place it on top of the pot.
• Place some ice cubes or an ice pack on top of the inverted cover. Introducing the cold temperature will force another state-of-matter change, turning the evaporated gas into liquid condensation. That condensation is distilled water.
• The glass bowl will collect the distilled water.
Fractional Distillation Using Physical Filtration through Charcoal, Ion Exchange, and Reverse Osmosis
The fractional filtration process can generally be divided into one of two categories: physical filtration and chemical filtration. Physical filtration, as the name would suggest, involves simply passing the water through materials that act like a sieve, leaving impurities behind and straining the "clean water" through.
In chemical filtration, the water passes through a material or membrane and in doing so, creates a chemical reaction, the by-product of which is the removal of impurities. There are a variety of physical and chemical filtration techniques (including combinations of the two methods), but we will focus here on three of the most popular: physical filtration through charcoal, ion exchange and reverse osmosis.
Charcoal is a softer form of carbon and is both materially solid and chemically porous, which makes it an ideal natural sieve for the physical filtration of water. Most commercially available water-pitcher based filters use charcoal as the mechanism for their filtration.
As water passes through the charcoal, many mineral impurities cannot pass through it, and are filtered out of the water that makes it into the pitcher.
The ion exchange method of purifying water is a good example of a chemical filtration process.
While the chemistry involved is quite complex, we can give a brief overview that demonstrates the broad mechanics of how chemical purification is achieved. In an ion exchange, a chemical reaction causes a "bad" element (impurity) to be replaced with a "good"element (pure water).
The most common example of an ion exchange system is in a water softener. Salt is used as a catalyst to spark the chemical reaction to trade "bad" elements for "good".
Impurities are removed and water is softened for the tap, showers and appliances throughout a household. It is worth noting that the salt never actually mixes with the water; it just factors into the chemical reaction caused by the water and equipment.
Reverse osmosis is also a physical filtration process. It sounds very complicated, but it can be explained fairly easily. Osmosis itself is the act of water moving through some sort of semi-permeable (meaning relatively solid, but with some porousness) membrane.
Due to the scientific principle of osmosis, water naturally moves from a higher concentrated form (purer water) to a lower concentrated form. That's why reverse osmosis is a purification technique.
By adding some sort of physical pressure generator into the technique, water is forced to move in the reverse direction, from less concentrated to more concentrated, therefore resulting in water that is more pure of minerals and chemicals. Most sink-based water filtration systems in homes and businesses employ a type of reverse osmosis system.
How long does Fractional Distillation Should Take?
First in fractional distillation, place it in a collection vessel under the distillate valve to catch your final alcohol product. A key to ensuring this step is followed properly is to make sure that all connections are tightly sealed and fit completely as they should. Connect your water source to your coolant input in order to cool the alcohol vapor. As the alcohol substance cools in temperature, it condenses into liquid ethanol. This liquid ethanol will dip into your collection vessel, hence, the collection vessel. Next, you will siphon your ethanol solution into your still. This step is very important, as you must avoid siphoning the bottom of your mash where the yeast is collected.
A siphon properly works by placing the shorter leg into the container above the longer leg that is placed into the lower one below. This allows the liquid to siphon into the shorter leg and into the longer leg by atmospheric pressure. Next, you will bring the solution to a boil slowly and begin running cold water once the solution reaches a temperature between 50 and 60 degrees Celsius or 122 to 140 degrees Fahrenheit. The distillation will begin when clear drops of liquid start to leave the spout and enter the collection vessel. The key to distillation is to discard the first 100mL of liquid (3.38 fluid oz.). This is referred to as "the head" and is removed for safety precautions because it contains deathly methanol. Next up, collect the following distilled liquid of 2-3L. Once the temperature reaches 96 degrees Celsius or 204.8 degrees Fahrenheit, stop collecting. First, turn off the heat source and then turn off the cold water. Open the collection vessel's lid to avoid creating a vacuum inside the still.
Benefits of Fractional Distillation
Now that we've clearly defined the different techniques to produce distilled water vs. purified water, we can examine the advantages and disadvantages of each method.
Cost
As you might imagine, distillation on any scale takes longer and involves more equipment than filtration. As the saying goes, "time is money" and therefore, distilled water tends to be more expensive than purified water.
Many companies sell home water filtration and purification system-usually a Reverse Osmosis system that can be installed under a kitchen sink. These companies are quick to correctly point out that these systems are generally quite affordable, as opposed to purchasing all the equipment required to distill water on a large scale.
While charcoal filtration pitchers are probably the most affordable purification system commercially available, they vary considerably in the amount of impurities they can fully remove. Even the best filters on the market cannot remove impurities at the level that reverse osmosis systems can.
Additionally, as filters wear out, they need to be replaced or they no longer serve their function as effectively. Over time, if a filter is not replaced, it can actually make the water in its pitcher less clear.
Taste
Taste is by its very nature subjective, so it's hard to definitively say that either distilled or purified water is better tasting. The bottled water you buy at a convenience store or from a vending machine is typically purified rather than distilled, which may lead some to assume purified water tastes better.
This is not necessarily the case, however, as different brands use different purification processes. Also, while specific impurities have to be at very low levels for water to be considered purified, some companies may place small amounts of harmless additives to improve flavor, or remove additional chemicals such as chlorine to create a more palatable taste.
Household Uses
It's interesting to note that many people think distilled water is not drinkable because it is commonly recommended for use in machines like irons, vaporizers, and humidifiers. The reason distilled water works so well with those devices is that the amount of clogging mineral buildup is reduced significantly when distilled water is used instead of tap water.
Distilled water can be used to safely fill and replace water in aquariums. It can also be beneficial to "top off" certain types of car batteries. Some people also extoll the virtues of using distilled water to make ice cubes, as they tend to be exceptionally clear.
Health Advantages
You may be surprised to learn that neither distilled nor purified water is intrinsically better for you. Arguments can be made for either, but ultimately both have had a large amount of impurities removed. They are both considerably better for you than drinking directly out of a faucet or a stream.
You should now have a better understanding of the differences between distilled water and purified water. While they undergo very different processes, the end goal is the same: mineral and chemical impurities are removed to provide clean, pure water.
Distilled water has many practical household uses for machines and appliances since distillation reduces the amount of minerals that build up around faucets or vents. Purified water may have more flavor and cost less.
While no technique can provide completely pure water, either is safer, cleaner alternative to unfiltered water.
The Materials Used for Fractional Distillation
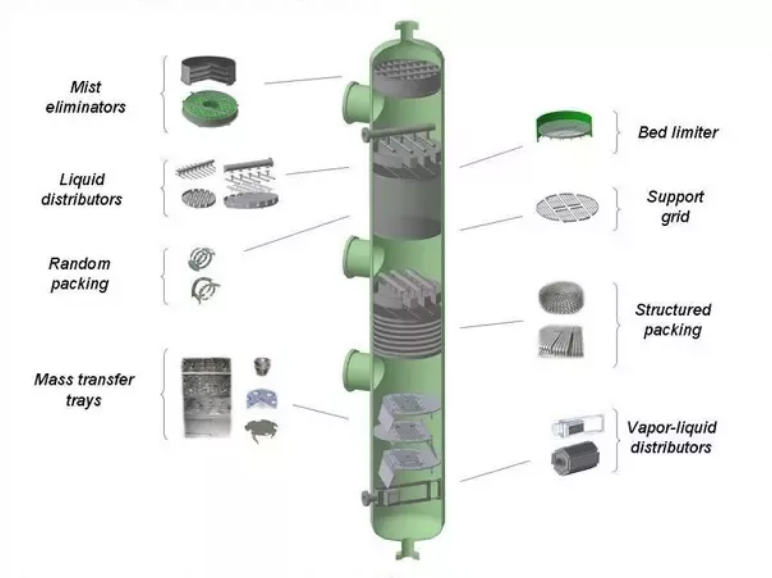
Tower Packing
If we look it carefully, tower packing uses materials with tower fill that people use for cooling towers. It gives high surface volume then condenses steam without the presence of air flow restriction. Tower packing is the product of combined solid gasses from many shapes.
If contained in a cylindrical container, there will be a larger surface area for both the liquids and all the gases. Some applications that is included in the process of tower packing are catalytic gas scrubbing, acid gas scrubbing, precipitation, mist elimination, aeration, and particulate removal.
Structured Packing
This is said to be as the range of necessary material that are designed and made for the purposes especially for distillation process and column absorptions from chemically-based reactors. Structured packing consists of corrugates pieces of metal gauzes and plates which are created to forcefully sustain fluid on constricted and complicated locations within the container column.
The result of structured packing is the honeycomb with the flow inclined in many channels that produces high surface areas with resistances from low gas flow. These are made to maximize the amount of liquid spreading in the applications which has lower pressure areas and low rates of irrigation.
Random packing
Specifically, random packing is the act of creating packs of columns that are used for distillation. These are used with random fits that are used for filtration with materials that are used to optimize the location areas. The reactants then interacts with each other as random packing minimizes the complex construction of each columns.
It offers the increase in the surface area from each column which results in the efficiency of the process. The reduction of the height from each amount is the result of this process with a considerable amount.
Pall Ring
As of the best known and common form of packing, the pall is considered to provide materials and products for the transfer of operations including the metal and plastic tower packs. Pall ring is done through the process of minimization of contours and other crevices that causes the liquid to hold-up and carry out potential entertainment.
The walls of these cylindrical materials are then opened and bent inwards to allow a capacity which is greater than the standard ones. Pall ring maintains and even distribution and the channeling of resistance tendencies.
Raschig Rings
As part of the process, the Raschig rings are the cylindrical tubes which has its own unique equal length that are utilized among the various chemical processes. Moreover, these are pieces of tubular materials which has an equal measurement in terms of diameter and length.
In the distillation process, these are used for packed bed and columns and other processes in the chemical engineering field. Raschig rings comes as a ceramic material or maybe a metal that gives larger surface areas within the column for the sake of gas and liquid vapor interactions.
The Fractional Distillation Products

The fractional distillation products you buy at the store is created in essentially the same way, just using massive machines in an industrial setting. It is important to note that most of the impurities typically found in tap water have a lower boiling point than water, which is why evaporation eliminates so many of them.
However, some have a higher boiling point than water, which is why the process of distillation is more effective than simply boiling water and letting it cool. Although they have been used for their varied medicinal purposes for thousands of years, essential oils have gained heightened popularity in recent years.
Many companies have emerged in the essential oils market, touting the benefits and purity of their products. With so many options to choose from, you need an essential oils guide to separate the unadulterated oils from the ones that are not.
We did exactly that. After investigating numerous essential oils companies, researching each of their products, and narrowing the list down, we created the following essential oils guide, just for you.
Essential Oils
Particularly, essential oils are the extracted, aromatic concentrates of various plant compounds. They may be extracted via distillation or through a mechanical process. Most essential oils should be diffused as aromatherapy or applied to the skin rather than ingested.
A few essential oils may be taken orally, but do this in moderation and always consult with your healthcare practitioner first. When rubbing essentials oils into the skin, dilute the oil with a carrier oil to ensure it does not irritate the area of application.
Each of the products on our essential oils guide serves an array of uses. Whether you suffer from anxiety, acne, and bug bites, insomnia, or beyond, you have lots of choices to pick from.
If you want to keep your house extra clean or add some delightful scents in a diffuser to keep the air smelling fresh, there are quite a few selections for you too.
Rum and Wines
Rum has been around since the 17th century. It originated in the 17th century in the Caribbean by way of sugar farmers who had a serious industrial waste problem on their hands.
These farmers produced sugar by crushing the sugar cane, boiling the leftover juices, and then leaving the boiled syrup to cure in clay pots. They use the early process of fractional distillation.We know the liquid leftover as molasses. In the 17th century, there was no common use for the liquid sugary goo. Slaves and livestock ended up eating the molasses but primarily this liquid goo was a waste that no one knew what to do with. Someone then discovered that you could mix the liquid during its initial boiling and fermenting stage that you could create a serviceable starting point to begin the distillation of rum. To whoever discovered this, we thank you! If we fast-forward to today and the current state of rum, we know that molasses is no longer an unwanted good and rum sales in the United States alone are upwards of over two billion in a standard year.
It is nice to know where it came from though, and we are thankful for that liquid sugar goo that is now the yummy liquid we call rum. If you really want to be extra when it comes to making your homemade rum, age the product in oak or toasted oak barrels. If you do not have access to the luxurious world of aged barrels, do not fret. You can use toasted oak chips or something similar to produce a similar taste. Filter your rum through a handy cheesecloth or strainer to remove any wood chips from your finished rum product. For the best tasting homemade rum, you will use distilled water to dilute your alcohol to enjoyable levels. Aim for a percentage of alcohol around 45%, which is common in drinkable alcohols. Some people love to get extra crafty and add cinnamon, ginger or cloves to their rum and soaking them for one to two weeks to add an extra flavor boost.
Synthetic Oil
Synthetic oil is just like any other oil, but it is, to some extent, man-made from fractional distillation. It is made from a base oil, power additives and carrier oil that enforces an even distribution of the synthetics additives.
Traditional motor oil and synthetics are made from refining oil. Most synthetic oil begins with highly refined crude oil pumped from deep underground. Again, this is the same source of conventional oil.
However, other synthetics are made from artificially-made compounds or a synthetic, as in a base oil. The main difference between traditional oil and synthetics is the level of refinement.
Base oil used in making synthetics falls into one of several oil grades from mineral oils. The mineral oils are typically extracted, from crude (Grade I and II) to fully-artificial, compound-based oils (Group V).
All oil grades are manufactured using additives, which increase performance significantly. The refinement process and ingredients used result in scientific control over the size of molecules and purity.
This lowers friction and reduces sludge while increasing performance. Synthetic oil was first fabricated in 1929.
Due to technological advances and access to resources, it can be refined to accommodate high-performance vehicles, jets and everyday consumer vehicles. The 1970 American energy crisis caused an enormous effort to improve fuel economy, and better synthetics were adopted.
Advantages of Synthetic Oil
There have been technological advantages regarding synthetic oil. They are:
• Less friction because of uniform molecular size
• Cleaner oil with negligible impurities
• Functions better in cold and extreme weather
• Protection-enhancing additives that can clean engines
• Deposits reduced by the refinement process
Conclusion
That is all we have for now about all you need to know about Fractional Distillation.
I hope you gained a lot from the guide.
If you have any questions, feel free to leave a comment below.









